Power and Type I Error Calculations
Alexandra Erdmann, Holger Löwe
Source:vignettes/trialplanning.Rmd
trialplanning.RmdIntroduction
This vignette demonstrates how to use simIDM for trial
design planning with a simple example. We will show how to estimate type
I errors and statistical power from simulations to optimize study
design, details can be found in Erdmann,
Beyersmann, and Rufibach (2023). Jointly modeling the endpoints
PFS and OS with the illness-death model has two major advantages: - We
properly account for the correlation between PFS and OS, - The
assumption of proportional hazards is not required.
OS is defined as the time to reach the absorbing state death, and PFS
is defined as the time to reach the absorbing state death
or the intermediate state progression, whichever occurs
first. Figure 1 shows the multistate model with the corresponding
transition hazards. In the vignette, we show how to estimate type I
errors and statistical power from simulations and give an idea on how
this can be used to plan complex study trials.
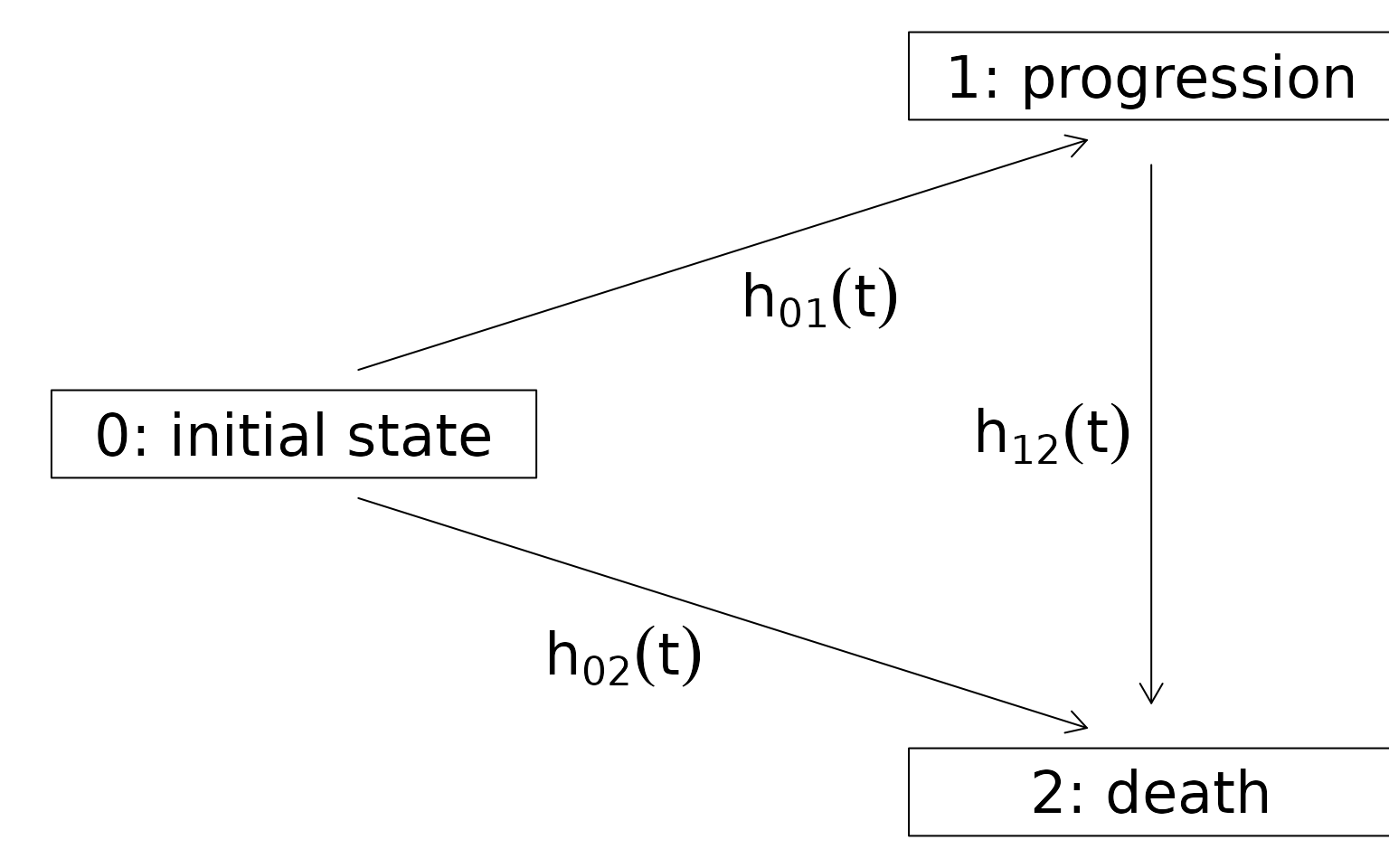
Figure 1 - Multistate model with indermediate state progession and absorbing state death
Scenario - PFS and OS as Co-primary Endpoints
We consider the following study design:
- PFS and OS as co-primary endpoints with one final analysis each, i.e. for a successful trial all endpoints need to be significant.
- Treatment vs. control group, 1:1 randomization ratio
- Global significance level of 5 %
- The standard log-rank test is used to assess the null hypothesis of equal survival functions in both groups for PFS and OS, respectively.
- Statistical power to detect a difference between the groups should be 80 % for each endpoint
- 5 % drop-out within 12 time units
- Accrual of 100 patients per time unit
Using the multistate model approach implies that trial planning is based on assumptions on the three transition hazards in each arm, i.e. six hazards in total. In our example scenario, we assume that all six transition hazards are constant and a small effect of the treatment on hazards from the initial state to death. Transition hazards are tuned such that median time until a PFS event is 0.87 time units in the control arm and 1.2 time units in the treatment arm. Median time until an OS event is 1.76 time units in the control group and 2.1 time units in the treatment group.
Figure 2 shows the transition hazards, survival functions, hazard functions and hazard ratios for both endpoints.
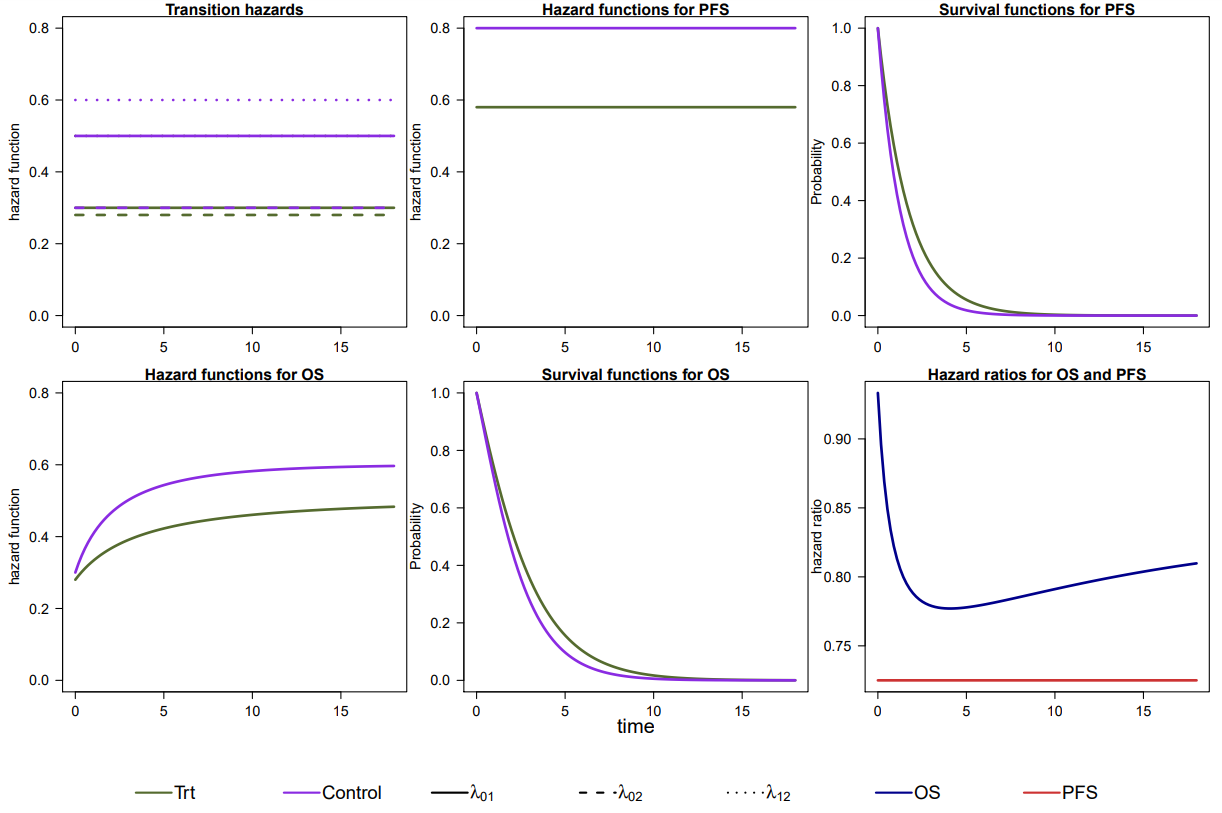
The transition hazards are specified as follows:
library(simIDM)
transitionTrt <- exponential_transition(h01 = 0.3, h02 = 0.28, h12 = 0.5)
transitionPl <- exponential_transition(h01 = 0.5, h02 = 0.3, h12 = 0.6)
transitionList <- list(transitionPl, transitionTrt)The package provides functions that return the values of the PFS or OS survival functions for given transition hazards (Constant, Weibull or Piecewise Constant) and pre-specified time points.
timepoints <- c(0, 0.1, 0.3, 0.7, 1, 5)
# OS Survival function for Constant transition hazards:
ExpSurvOS(timepoints, h01 = 0.2, h02 = 0.4, h12 = 0.1)
#> [1] 1.0000000 0.9610787 0.8893403 0.7671856 0.6912219 0.2724845
# OS Survival function for Weibull transition hazards:
WeibSurvOS(timepoints, h01 = 0.2, h02 = 0.5, h12 = 2.1, p01 = 1.2, p02 = 0.9, p12 = 1)
#> [1] 1.00000000 0.93822237 0.83706585 0.66353708 0.55296799 0.03684786
# OS Survival function for Piecewise Constant transition hazards:
PWCsurvOS(timepoints,
h01 = c(0.3, 0.5), h02 = c(0.5, 0.8), h12 = c(0.7, 1),
pw01 = c(0, 4), pw02 = c(0, 8), pw12 = c(0, 3)
)
#> [1] 1.00000000 0.95094877 0.85849702 0.69546105 0.59109798 0.03945673There are also functions for PFS survival functions available:
timepoints <- c(0, 0.1, 0.3, 0.7, 1, 5)
# PFS Survival function for Constant transition hazards:
ExpSurvPFS(timepoints, h01 = 0.2, h02 = 0.4)
#> [1] 1.00000000 0.94176453 0.83527021 0.65704682 0.54881164 0.04978707
# PFS Survival function for Weibull transition hazards:
WeibSurvPFS(timepoints, h01 = 0.2, h02 = 0.5, p01 = 1.2, p02 = 0.9)
#> [1] 1.00000000 0.92721907 0.80545180 0.61074857 0.49658530 0.02995439
# PFS Survival function for Piecewise Constant transition hazards:
PWCsurvPFS(timepoints,
h01 = c(0.3, 0.5), h02 = c(0.5, 0.8),
pw01 = c(0, 4), pw02 = c(0, 8)
)
#> [1] 1.00000000 0.92311635 0.78662786 0.57120906 0.44932896 0.01499558For PFS, the hazard ratio under is known by specification:
hTrtPFS <- sum(unlist(transitionTrt$hazards[1:2]))
hPlPFS <- sum(unlist(transitionPl$hazards[1:2]))
hRatioPFS <- hTrtPFS / hPlPFS
hRatioPFS
#> [1] 0.725For OS, the ratio of hazard functions is not necessarily constant. An
averaged HR can be calculated using avgHRExpOS:
hRatioOS <- avgHRExpOS(transitionByArm = transitionList, alpha = 0.5, upper = 1000)
hRatioOS
#> [1] 0.8072368Type I Error - Simulation Under
The type I error can be estimated empirically by simulating clinical
trials under
.
To achieve this, we set the transition hazards of the treatment group to
match those of the control group. Then, we use
getClinicalTrials() to generate a large number of simulated
trials. We will use 100 iterations here. For applications, to achieve
satisfactory precision in estimates of type I error, a higher number
(e.g. 10,000) is recommended.
transitionListNull <- list(transitionPl, transitionPl)
nRep <- 100
simNull <- getClinicalTrials(
nRep = nRep, nPat = c(800, 800), seed = 1238, datType = "1rowPatient",
transitionByArm = transitionListNull,
dropout = list(rate = 0.05, time = 12), accrual = list(param = "intensity", value = 100)
)Using the simulation, we can now identify critical log-rank test values for both PFS and OS to maintain a 5% global significance level. Initially, we allocate critical values so that the two-sided log-rank test has a 4% significance level for the OS endpoint and 1% for the PFS endpoint, effectively splitting the global significance level. Such a Bonferroni correction is widely used in trials with co-primary endpoints.
alphaOS <- 0.04
alphaPFS <- 0.01
criticalOS <- abs(qnorm(alphaOS / 2))
criticalPFS <- abs(qnorm(alphaPFS / 2))With the Schoenfeld approximation, preliminary sample sizes can be computed to get an idea of how many events are needed to achieve 80 % power:
library(rpact)
# Number of PFS events required for 80 % power via Schoenfeld:
schoenfeldPFS <- getSampleSizeSurvival(
lambda2 = hPlPFS, hazardRatio = hRatioPFS,
accrualTime = 0, accrualIntensity = 500,
maxNumberOfSubjects = 1000, sided = 1,
alpha = alphaPFS / 2, beta = 0.2
)
eventNumPFS <- ceiling(schoenfeldPFS$eventsFixed)
eventNumPFS
#> [1] 452
# Number of OS events required for 80 % power via Schoenfeld:
schoenfeldOS <- getSampleSizeSurvival(
lambda2 = hPlPFS, hazardRatio = hRatioOS,
accrualTime = 0, accrualIntensity = 500,
maxNumberOfSubjects = 1000, sided = 1,
alpha = alphaOS / 2, beta = 0.2
)
eventNumOS <- ceiling(schoenfeldOS$eventsFixed)
eventNumOS
#> [1] 732Using these critical values and required number of events for PFS and
OS, we can now determine the global type I error empirically by counting
the number of trials simulated under
with significant log-rank tests. The empirical type I error for each
endpoint is calculated as the proportion of trials with significant
log-rank tests, while the global Type I error is the proportion of
trials where at least one of PFS or OS with a significant log-rank test.
This can be done using empSignificant:
empSignificant(
simTrials = simNull,
criticalPFS = criticalPFS,
criticalOS = criticalOS,
eventNumPFS = eventNumPFS,
eventNumOS = eventNumOS
)
#> $significantPFS
#> [1] 0
#>
#> $significantOS
#> [1] 0.03
#>
#> $significantAtLeastOne
#> [1] 0.03
#>
#> $significantBoth
#> [1] 0In this example, the global type I error rate is 3%.
Sample size and power calculation - simulation under
Next, we simulate a large number of trials under to compute the empirical power:
simH1 <- getClinicalTrials(
nRep = nRep, nPat = c(800, 800), seed = 1238, datType = "1rowPatient",
transitionByArm = transitionList,
dropout = list(rate = 0.05, time = 12), accrual = list(param = "intensity", value = 100)
)The empirical power for each endpoint is the proportion of simulated trials with significant log-rank tests under . The multistate model approach allows us to easily estimate further interesting metrics, such as joint power, i.e. the probability that both endpoints in a trial are significant, if each endpoint is analyzed at its planned time-point.
empSignificant(
simTrials = simH1,
criticalPFS = criticalPFS,
criticalOS = criticalOS,
eventNumPFS = eventNumPFS,
eventNumOS = eventNumOS
)
#> $significantPFS
#> [1] 0.83
#>
#> $significantOS
#> [1] 0.75
#>
#> $significantAtLeastOne
#> [1] 0.9
#>
#> $significantBoth
#> [1] 0.68In this example, for the endpoint OS, the number of events has to be increased to obtain a power of 80 %. Similarly, we can reduce the number of events if the simulation shows that a trial design appears to be overpowered.
It is also possible to derive the median time at which a certain number of events are expected to occur and how many events of the second endpoint have occurred at that time on average.
# median time point for 329 PFS events to have occurred:
timePointsPFS <- lapply(simH1, getTimePoint,
eventNum = 329, typeEvent = "PFS",
byArm = FALSE
)
median(unlist(timePointsPFS))
#> [1] 2.912601
# median time point for 684 OS events to have occurred:
timePointsOS <- lapply(simH1, getTimePoint,
eventNum = 684, typeEvent = "OS",
byArm = FALSE
)
median(unlist(timePointsOS))
#> [1] 5.804041
# mean number of PFS events at time of OS analysis
eventsPFS <- lapply(
seq_along(timePointsPFS),
function(t) {
sum(simH1[[t]]$OSevent[(simH1[[t]]$OStime + simH1[[t]]$recruitTime) <= timePointsPFS[[t]]])
}
)
mean(unlist(eventsPFS))
#> [1] 219.31
# mean number of OS events at time of PFS analysis
eventsOS <- lapply(
seq_along(timePointsOS),
function(t) {
sum(simH1[[t]]$PFSevent[(simH1[[t]]$PFStime + simH1[[t]]$recruitTime) <= timePointsOS[[t]]])
}
)
mean(unlist(eventsOS))
#> [1] 864.08